product introduction
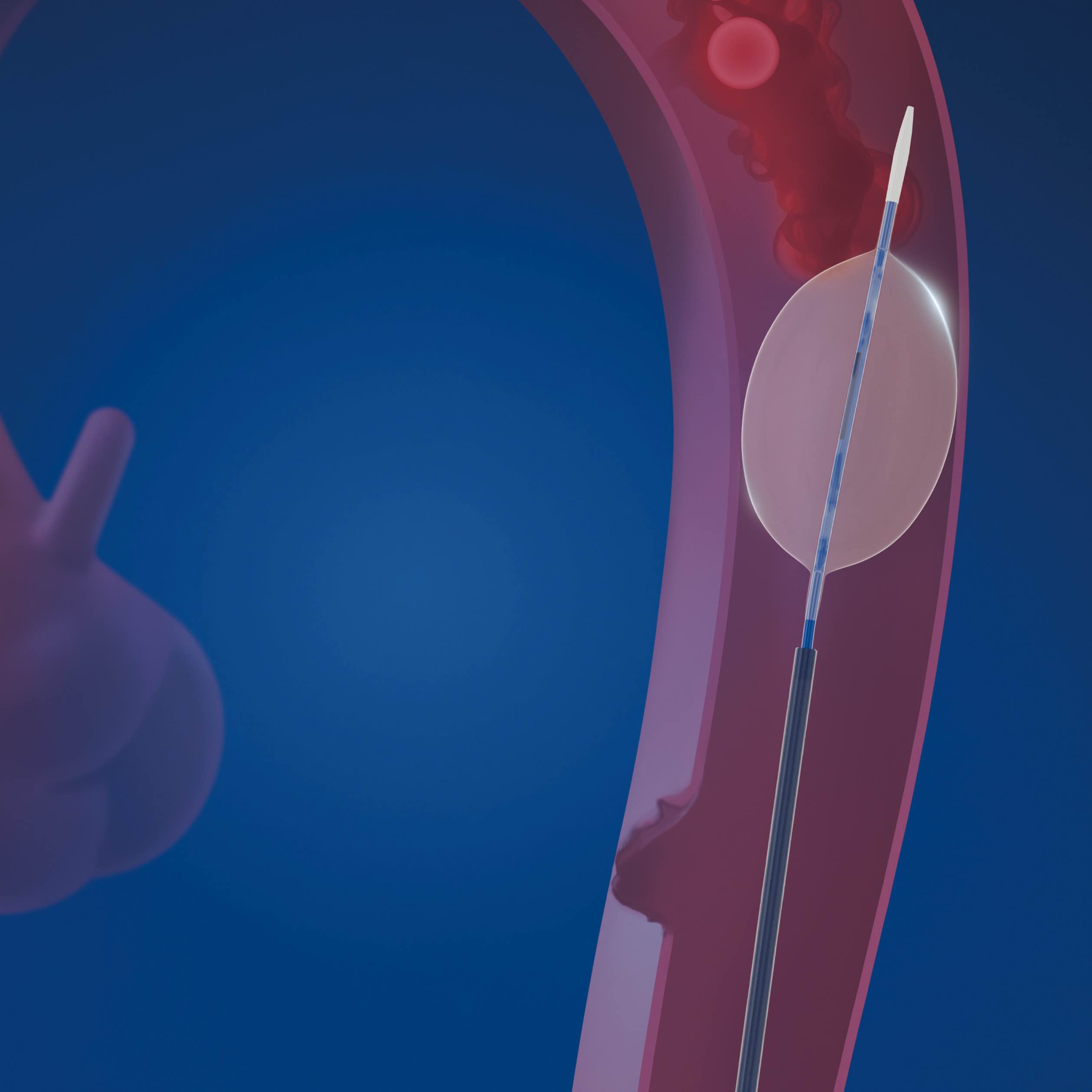
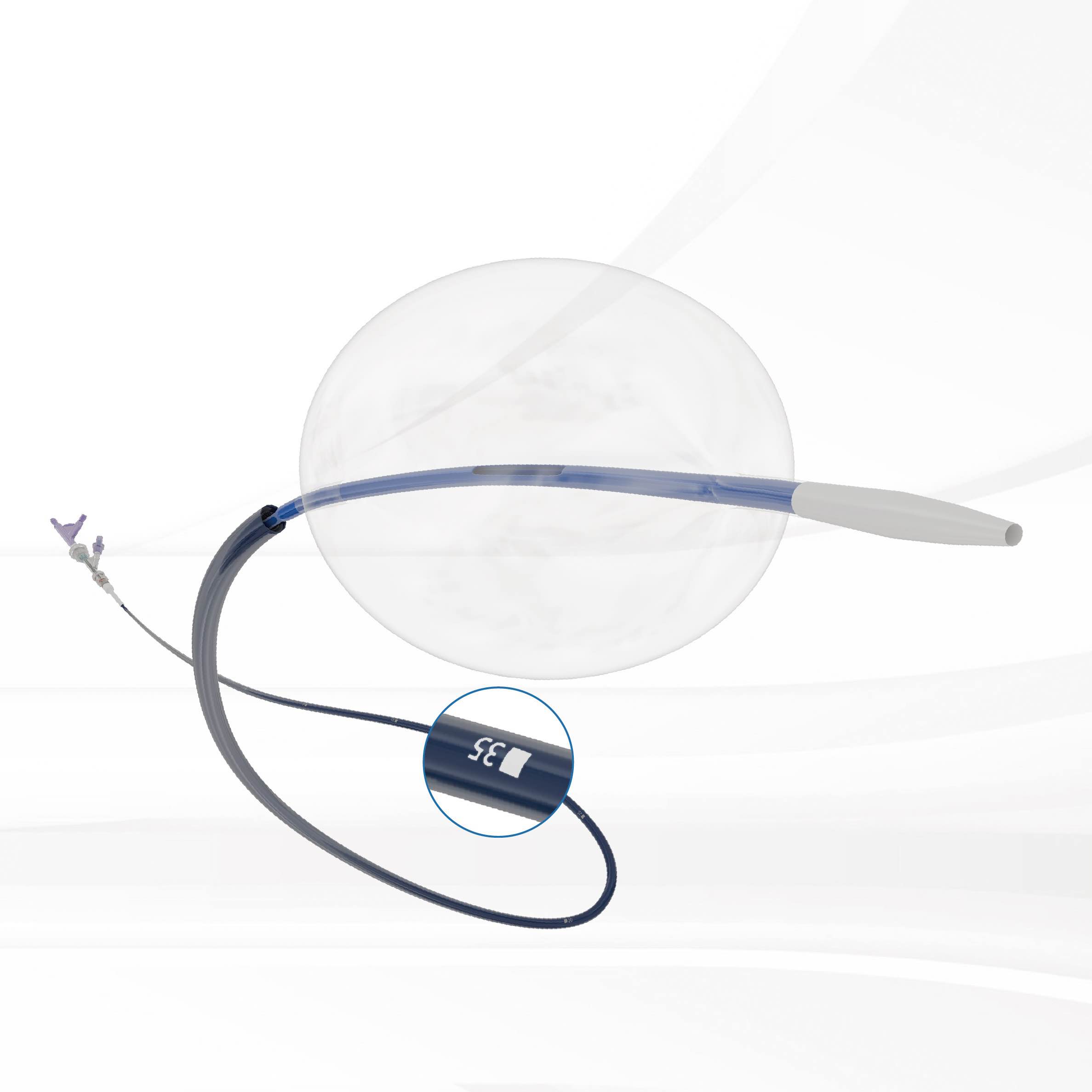
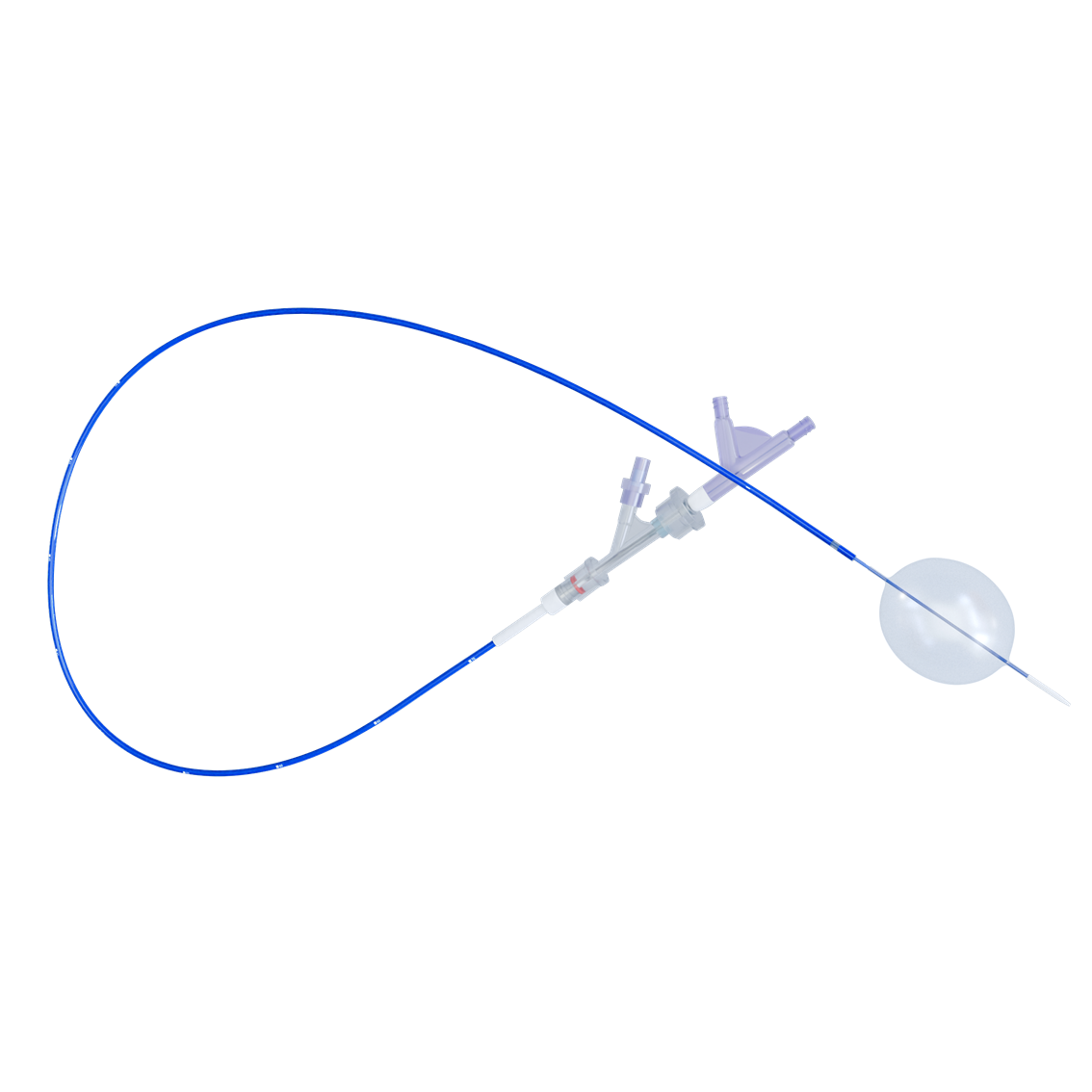
l-reboa™ aortic occlusion balloon catheter is indicated for temporary aortic blood flow occlusion. it is the first product specifically designed for resuscitative endovascular balloon occlusion of the aorta (reboa) in china. different from the traditional compliant balloon products to expand stent graft, the demand of rapid introduction, migration resistance and supportability are stricter for reboa balloon. hence, the integration of balloon catheter and 7f labeled long sheath successfully satisfies these demands. due to the unnecessary of additional introducer sheath, the operation is simplified and the rescue time is saved. the incidence of occlusion failure caused by balloon migration could be reduced by the rigidity and strong supportability of braided sheath. what’s more, under the emergency situation, accurate and quick positioning can be achieved by aligning the labels on the sheath leading to rapid treatment and reduction of mortality. lastly, a low profile sheath (7f) could reduce the incidence of vascular access-site complications.
● integration: the integration of balloon catheter and 7f labeled long sheath, unnecessary of additional introducer sheath and simplified operation
● stability: braided sheath, strong supportability and stable occlusion
● fast: accurate and quick positioning can be achieved by the labeled long sheath to rapid treatment
● low profile: 7f long sheath, small access-site complications
